Policosanol Research
Recent Policosanol Research Reported by Dr. Teresa Hill:1
Here is the exciting clinical research that has just been released. Policosanol was tested against statins and lowered LDL cholesterol as much as levostatin and pravastatin.
In this same clinical trial Lipitor and policosanol both significantly lowered cholesterol, but the policosanol significantly raised the HDL (good cholesterol). The policosanol group did not raise the liver enzymes like the Lipitor group did. Policosanol also helped to lower glucose, CPK and liver AST enzymes.
Previous Policosanol Research includes well designed
clinical trials
involving nearly 30,000 patients.
The most effective Policosanol, and the one used in the original research, is a natural supplement derived from sugar cane.
In clinical policosanol research trials of more than 30,000 persons it has been shown to significantly improve cholesterol ratios, and most importanly the good HDL cholesterol.
These well-designed policosanol research trials have included short and long-term, randomized, double-blind studies comparing policosanol to a placebo as well as double-blind comparative trials versus statin drugs.
In the studies Policosanol produced cholesterol-lowering effects within the first 6-8 weeks of use. At a daily dosage of 10-20 mg of policosanol at night, LDL cholesterol levels typically dropped by 20 to 30% within the first six months of therapy.
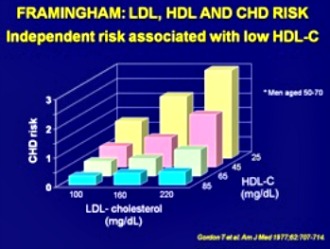 Framingham Study Shows Importance of Raising HDL Cholesterol
Framingham Study Shows Importance of Raising HDL Cholesterol
The research study concluded;"...risk for CAD increases sharply as HDL levels fall progressively below 40 mg/dL."
To cite the report: "For each 1 mg/dl drop of cholesterol there was an 11 percent increase in coronary and total mortality"
In policosanl research studies of HDL cholesterol levels (the protective cholesterol) typically increase by 15 to 25% after only two months of use.
The combined LDL reduction and HDL increase can produce dramatic improvements in the LDL to HDL ratio, which lowers risk of a heart attack or stroke!
POLICOSANOL RESEARCH DESCRIPTION and MECHANISMS
Policosanol is a mixture of fatty alcohols derived from the wax of sugarcane .(Saccharum officinarum, L.) These active substances work to lower cholesterol levels by several mechanisms including blocking the formation of cholesterol in the liver. The components of policosanol include 1-octacosanol, 1-dotriacontanol, 1-triacontanol, 1- tetracosanol, 1-tetratriacontanol, 1-hexacosanol, 1-heptacosanol and 1-nonacosanol.
CHOLESTEROL MANAGEMENT
Need a less technical, condensed, easier to understand "Readers Digest" version of the Policosanol Research?
Click on this Policosanol linkThe Policosanol Research confirms that policosanol is indicated as an adjunct to dietary and lifestyle recommendations to reduce elevated LDL-C and total cholesterol levels. Its primarily application is in type II hypercholesterolemia including IIa subtype (characterized by elevated total serum cholesterol and LDL-C levels) and IIb subtype (mixed hypercholesterolemia characterized by elevated total serum cholesterol, LDL-C andtriglyceride levels). Policosanol can also be used as an alternative to aspirin as an anti-platelet agent.
POLICOSANOL:PHARMACOKINETICS
Policosanol is rapidly absorbed based on radioactive absorption studies in experimental animals (rats, rabbits and monkeys) and humans.1,2 Peak levels have been achieved from 30 to 120 min after treatment in different animal species and humans. Radioactivity is mainly distributed in the liver while radioactivity levels in the systemic circulation are low.
This effect is an advantage for a cholesterol-lowering agent since the liver is the main organ for synthesis and regulation of cholesterol metabolism. Excretion studies in animals and human healthy volunteers have demonstrated that feces is the main route for radioactivity excretion after oral administration, urinary excretion is not relevant.
CLINICAL RESEARCH PHARMACOLOGICAL EFFECTS indicated
The pharmacological effects of policosanol based on the policosanol research experimental models can be summarized as follows:
- Policosanol produces a dose-dependent and significant reduction of serum total cholesterol and LDL-C levels. HDL-C values were also increased in a dose-dependent manner.
- Triglycerides are also significantly reduced, but the reduction is not dose-dependent.3,4,
- Policosanol Research shows policosanol lowers total cholesterol and LDL-C by:
- Inhibiting cholesterol synthesis at a point between the formation of acetate and mevalonate.5,6
- Exerting no direct inhibition on HMG-CoA reductase.5,6
- Significantly increasing LDL receptor dependent processing as demonstrated by increasing the incorporation of LDL into the hepatocyte and stimulating its catabolism.5,6
The Policosanol Research indicates that policosanol not only effectively decreases serum cholesterol levels,but also reduces the cholesterol content in different tissues such as liver, heart and fatty tissue.7
The cholesterol-lowering effects of policosanol are persistent and it does not lose its effect over time.
- Policosanol Research shows platelet aggregation by altering prostaglandin synthesis. Specifically, policosanol lowers serum levels of the pro-aggregatory thromboxane A2, while increasing the anti-aggregatory prostaglandin prostacyclin.8-10
- Policosanol prevents and reverses atherosclerotic lesions and thrombosis.11-15
- Policosanol prevents intimal thickening and smooth muscle cell proferation.16,17
- Policosanol is an effective antioxidant in preventing LDL oxidation.18,19
CLINICAL EFFICACY SHOWN IN POLICOSANOL RESEARCH
Policosanol is an agent to lower cholesterol with exceptional clinical documentation demonstrating efficacy, safety and tolerability in patients with type II hypercholesterolemia and in patients with secondary hypercholesterolemia associated to diabetes mellitus or nephrotic syndrome.
The policosanol research includes short and long-term, randomized, placebo-controlled and comparative studies versus statins (lovastatin, pravastatin and simvastatin), fibrates (bezafibrate and gemfibrozil), acipimox, and probucol involving nearly 3,000 subjects.
In these studies, policosanol in dosages ranging from 5 to 20 mg/day, has demonstrated significant improvements in LDL-C, total cholesterol, HDL-C, and the ratios of total cholesterol to HDL-C and LDL-C to HDL-C. Policosanol is shown to lower cholesterol within the first 6-8 weeks of use.
POLICOSANOL RESEARCH DOSAGE
The recommended starting dose is 10-20 mg once a day with the evening meal, since cholesterol biosynthesis is increased at night.
An evening dosage is preferred because most cholesterol manufacture occurs at night. As with other cholesterol-lowering therapies, dosage may be adjusted periodically according to changes in blood cholesterol levels.
At a daily dosage of 20 mg, LDL levels typically drop by 25-30% during the first 8-12 weeks of therapy. HDL cholesterol levels typically increase by 15 to 25% after only two months of use. The combined LDL reduction and HDL increase can produce dramatic improvements in the LDL to HDL ratio.
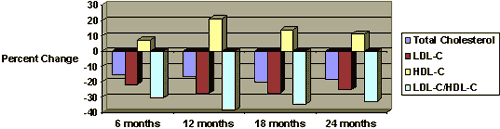
Figure 1. Policosanol research indicates that the lipid-lowering effects of policosanol are dose-dependent (% changes compared to placebo in eight-week treatment periods).22

Policosanol Research indicates that these improvements in lipid profiles compare quite favorably to results observed with statin drugs. From comparative studies it can be concluded that 10 mg of policosanol is equivalent in efficacy to 20 mg of lovastatin, 10 mg pravastatin, and 10 mg of simvastatin.
But, while these drugs have well-known side effects, the policosanol research indicates that it is completely safe. Policosanol has not been shown to produce any adverse drug interaction as well and it can be used in diabetics, elderly subjects, and even in patients with impaired liver function or severe liver damage without fear of side effect.
In addition to its effects on cholesterol levels, policosanol also exerts additional positive effects in the battle against atherosclerosis.
It prevents excessive platelet aggregation without effecting coagulation, prevents smooth muscle cell proliferation into the intima of the artery, and exerts good antioxidant effects in preventing against LDL oxidation.
Figure 2. The efficacy of 10 mg of policosanol daily is maintained in long-term therapy (comparison vs. placebo).29
DOUBLE-BLIND POLICOSANOL RESEARCH STUDIES VS. CHOLESTEROL-LOWERING DRUGS
In 1997 Policosanol research compared statin drugs lovastatin (Mevacor), simvastatin (Zocor) and pravastatin (Pravachol) with policosanol.
Footnote. Since that time new statin drugs have come to the market. Atorvastatin (Lipitor), rosuvastatin (Crestor) and a combination drug called Vytorin which consists of simvastatin (Zocor) and eztimibe. Although comparison studies have not been conducted on these newer statin drugs they are similar in function to their predecessors.
Vs. Lovastatin (Mevacor)
Policosanol administered for eight weeks at 10 mg day has shown a similar efficacy to lovastatin administered at 20 mg/day.31,32Both drugs produced similar decreases in LDL-C levels, while lovastatin was slightly more effective than policosanol in reducing total cholesterol. However, the reason is thatpolicosanol, but not lovastatin, significantly increased HDL-C levels in these studies.
Policosanol raised HDL levels by over 17% from baseline values while lovastatin actually decreased HDL-C levels slightly.
The policosanol research shows that there is another advantage. It has no hepatotoxic effect. In other words it is easy on the liver. Lovastatin significantly, but moderately, increased serum transaminases and creatine phosphokinase values while policosanol did not. Other side effects were also noted in lovastatin-treated patients but not in policosanol.
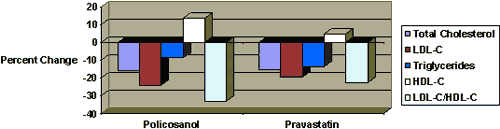
Vs. Pravastatin (Pravachol)
Policosanol administered at 10 mg/day was compared with the same dosage of pravastatin for eight weeks.33 The policosanol group demonstrated greater percent changes of LDL-C and HDL-C than the pravastatin group. Side effects were more frequent in the pravastatin group than in policosanol group.While pravastatin produced a significant increase the serum levels of alanine and aspartate aminotransferase (ALT and AST, respectively), policosanol exhibited no hepatotoxicity.
Policosanol Research Comparsion of Simvastatin (Zocor)
Policosanol and Simvastatin were found to be equally effective at dosages of 10 mg/day for eight weeks in patients with type-II hypercholesterolemia.34,35In patients with type-II hypercholesterolemia and concomitant NIDDM, policosanol, but not Simvastatin, significantly increased HDL-C levels.35 Again, more adverse experiences were and have been reported in simvastatin treated patients than in policosanol treated patients.
Policosanol Significantly Reduced Lp(a)
A comparative double blind clinical trial versus acipimox for eight weeks in type II hypercholesterolemic patients has shown that policosanol is more effective than acipimox in reducing LDL-C and total cholesterol.39
In addition, the policosanol research indicates that serum Lp(a) levels were significantly reduced by policosanol treatment both in the whole study population (32.6 % reduction) as well as in the stratum showing initial high Lp(a) levels (> 30 mg/dl) (57.4 % reduction).
Lp(a) is a plasma lipoprotein with a structure and composition that closely resembles LDL, but with an additional molecule of an adhesive protein called apolipoprotein (a).
Elevated plasma levels of Lp(a) are an independent risk factor for coronary heart disease, particularly in patients with elevated LDL cholesterol levels.
A high level of Lp(a) has been shown to carry a ten times greater risk for heart disease than an elevated LDL cholesterol level. That is because LDL on its own lacks the adhesive apolipoprotein (a). As a result, LDL does not easily stick to the walls of the artery. Levels of Lp(a) below 20 mg/dl are associated with a low risk for heart disease; levels between 20 and 40 mg/dl a moderate risk; and levels above 40 mg/dl an extremely high risk for heart disease.
DOUBLE-BLIND POLICOSANOL RESEARCH IN SPECIAL POPULATIONS
Policosanol Research in Diabetics
Policosanol administered to non-insulin dependent diabetes mellitus (NIDDM) patients with type-II hypercholesterolemia significantly lowered LDL-C, serum total cholesterol and atherogenic ratios, while increasing HDL-C levels.
In addition, policosanol does not impair glycemic control in diabetic patients as assessed through the evaluation of its effects on blood glucose and glycosylated hemoglobin (HgbA1c) values.41,42
| Table 2. Effect of policosanol on serum lipid profile of patients with NIDDM.41 | |||
| Treatment | Baseline | 12 weeks | Percent change |
| Total cholesterol (mmol/L) | |||
| Policosanol Placebo |
7.51 7.94 |
5.35 8.01 |
-28.9 +0.4 |
| LDL-C (mmol/L) | |||
| Policosanol Placebo |
5.27 5.32 |
3.05 5.56 |
-44.4 +3.4 |
| HDL-C (mmol/L) | |||
| Policosanol Placebo |
1.47 1.51 |
1.58 1.52 |
+23.5 +0.7 |
| Triglycerides (mmol/L) | |||
| Policosanol Placebo |
2.06 2.45 |
1.96 2.11 |
-2.4 +6.5 |
| Total cholesterol to HDL-C | |||
| Policosanol Placebo |
5.88 5.72 |
3.52 6.03 |
-38.3 +3.8 |
| LDL-C to HDL-C | |||
| Policosanol Placebo |
4.25 3.99 |
2.01 4.16 |
-51.6 +2.9 |
Policosanol Research in Hypertensive patients (high blood pressure)
Policosanol significantly reduced LDL-C (-19.1%), total cholesterol (-13%) and the ratios of cholesterol to HDL-C (-20%) and LDL-C to HDL-C (-24.2%) in hypertensive patients with hypercholesterolemia, while significantly increasing HDL-C levels (+17.1%).43
After 12 months of therapy policosanol significantly lowered systolic pressure (-10 mm Hg), while in the placebo group the values remained unchanged.
Many of the patients were on beta-blockers and diuretics, two classes of drugs known to adversely impact blood lipid levels.
Policosanol Research in Elderly Patients
Policosanol administered for short or long-term in patients over the age of 60 years with hypercholesterolemia has been effective, safe and well tolerated.44,45
In this population policosanol has a similar efficacy profile to that observed in patients below 60 years old. Table 3 summarizes the main results obtained at months 6 and 12 in a long-term study performed in elderly patients.
Of particular importance in this population is the fact thatno drug-related adverse experiences have been shown.
Elderly patients are at risk for such problems due to impaired renal and hepatic clearance as well as a high coexistence of concomitant diseases and of medications consumption are present.
| Table 3. Effect of policosanol on the serum lipid profile in elderly patients with hypercholesterolemia | ||||
| Treatment | Baseline | 6 months | 12 months | Percent change |
| Total cholesterol (mmol/L) | ||||
| Policosanol Placebo |
7.68 7.33 |
6.67 7.46 |
6.43 7.57 |
-16.4 +0.03 |
| LDL-C (mmol/L) | ||||
| Policosanol Placebo |
5.40 4.99 |
4.34 5.22 |
4.10 5.24 |
-24.1 +4.8 |
| HDL-C (mmol/L) | ||||
| Policosanol Placebo |
1.28 1.28 |
1.28 1.25 |
1.36 1.25 |
+5.9 -2.4 |
Patients with Type II Hypercholesterolemia and Disturbances of Hepatic Function
Policosanol Research on The efficacy pattern of policosanol in patients with type II hypercholesterolemia and concomitant disturbances of hepatic function is similar to that shown in hypercholesterolemic patients without impairment of liver function.46
Policosanol reduced total cholesterol (-13.6%), LDL-C (-19.1%), LDL-C to HDL-C ratio (-25.5%) and raised HDL-C (+11.5%).
In addition, policosanol was shown to reduce levels of alanine aminotransferase (ALT) and gamma-glutamyltranspeptidase (GGT) toward normal values.
Policosanol Research in the Nephrotic Syndrome
Policosanol reduced effectively total cholesterol, LDL-C and triglycerides values while increasing HDL-C levels in patients with the nephrotic syndrome without adversely affecting renal function.47
POLICOSANOL RESEARCH: ANTI-PLATELET EFFECTS
Policosanol reduces platelet aggregation by altering prostaglandin synthesis. Specifically, policosanol lowers serum levels of the pro-aggregatory thromboxane A2, while increasing the anti-aggregatory prostaglandin prostacyclin.
Clinical trials in humans have shown that policosanol significantly inhibits platelet aggregation without affecting coagulation parameters.9-11 Policosanol's effects on platelet aggregation compare quite favorably to low-dose aspirin.48
| Table 5. Effects of policosanol (10 mg/day) or placebo on platelet aggregation in 30 healthy volunteers | |||
| Baseline | After treatment | Difference | |
| Arachidonic acid 0.5 M | |||
| Policosanol Placebo |
68.5 70.0 |
43.3 72.5 |
-25.2 +2.6 |
| Epinephrine 1.25 x 10-5 M | |||
| Policosanol Placebo |
63.8 59.1 |
46.0 62.8 |
-17.8 +3.8 |
| Collagen 0.5 mcg/ml | |||
| Policosanol Placebo |
67.7 64.0 |
51.8 67.5 |
-16.0 +3.6 |
| ADP 2 x 10-6 M | |||
| Policosanol Placebo |
56.7 54.7 |
50.9 57.9 |
-5.8 +3.2 |
POLICOSANOL RESEARCH SHOWS ANGINA IMPROVEMENT
Policosanol was shown to improve the clinical evolution, and exercise-ECG testing responses of coronary heart disease (CHD) patients with myocardial ischemia, documented by exercise myocardial perfusion scintigraphy.49 In the double-blind study, 15 patients were treated with 5 mg of policosanol twice daily; another 15 patients were administered the same dose plus 125 mg aspirin; and the other 15 patients received placebo plus equal aspirin dose.
They were followed for 20 months, previous baseline observations, with treadmill exercise-ECG, besides serum lipid test. Beneficial changes on proportions among the 2 policosanol groups and the placebo group, showed an increment on functional capacity class, a decrement on rest and exercise angina, and a significant decrease in cardiac events, and in ischemic ST segment response, especially in the policosanol plus aspirin group.
POLICOSANOL RESEARCH: SIDE EFFECTS, SAFETY AND TOXICOLOGY
Policosanol exhibits an exemplary safety profile. In all controlled studies, policosanol has exerted no negative effect of any clinical or laboratory parameter. Side effects were comparable to a placebo.
The withdrawal rate for policosanol in short and long-term clinical studies was comparable or even lesser than that of placebo; only 0.2 % policosanol-patients withdrew before conclusion of the study as a result of an adverse experience, compared with 0.6 % of placebo patients. Comparative studies have shown a dropout rate due to side effects of 0.9% in policosanol-treated patients compared with a 4.4% rate for those treated with other lipid-lowering drugs (e.g., statins, fibrates, probucol, and acipimox).
In a large post marketing policosanol research surveillance study, the tolerability of policosanol was assessed in 27,879 patients (17,225 patients for two years and 10,654 patients for four years). All of the patients were treated for at least one month. During the study, 86 patients (0.31%) reported adverse effects, the most frequent of which was weight loss.
A single dose (1,000 mg/day) as much as 50 times the maximum recommended dose (20 mg/day) administered to healthy volunteers produced no adverse reaction, hence no over dosage symptoms have been detected.
Animal policosanol research studies demonstrate the policosanol is virtually non-toxic as the oral LD50 in rats, mice, rabbits and dogs was > 5 000 mg/kg. Body weight gain, behavioral assays, as well as biochemical and hematological determinations in surviving animals at the end of the test (14 days) did not reveal differences between treated and control groups. Moreover, weight organ analysis and histopathological study did not reveal differences between groups.51,52
The effects of successive dosage increases of policosanol administered orally to Macaca arctoides monkeys demonstrated that even the highest dose administered (500 mg/kg) Policosanol was tolerated. Similar results have been shown oral subchronic and chronic toxicity models in rats, dogs, and monkeys.53-55Policosanol did not produce any adverse effects on fertility and reproduction in animal studies, nor has it exerted any mutagenic or carcinogenic effects.56-60 Specifically, policosanol administered orally up to 500 and 1000 mg/kg during the organogenesis period did not produce embryotoxic nor teratogenic effects in rats or rabbits and a multigenerational study did not shown any toxicity.
CONTRAINDICATIONS TO POLICOSANOL
PregnancyAlthough policosanol neither induced teratogenic effects in rats or rabbits nor affected rat fertility and reproduction, the treatment is not allowed to use in pregnant women. The reason for this restriction is that cholesterol and associated metabolic products are required for an adequate fetal development. Since hypercholesterolemia and atherosclerosis are chronic diseases, the suspension of lipid-lowering therapy for 9 months cannot be considered as an additional coronary risk factor.
Lactation - It is not known whether the product or some active metabolite is excreted via the human milk during nursing, therefore therapy should be discontinued during lactation.
Pediatric use - Efficacy and safety of policosanol in children has not been well established. Thus, treatment of children with policosanol is not recommended at the present.
POLICOSANOL RESEARCH STUDIES:DRUG INTERACTIONS
ASPIRIN
The policosanol research has demonstrated synergism with the anti-platelet properties of aspirin in experimental animal models and healthy human volunteers as well as in different experimental animal models of ischemia and thrombosis.Pretreatment with policosanol inhibited aspirin-induced gastric ulcer in experimental animals.
Anticoagulants
Single or repeated doses of policosanol administered orally did not significantly affect fibrinolytic activity or bleeding time in rats. In these studies interaction between policosanol and heparin or warfarin have been ruled out.61
Antipyrine and theophylline
Antipyrine is a model drug used to investigate interaction with drugs metabolized by liver microsomal enzymes (the P-450 system). Policosanol administered orally to Beagle dogs for 3 to 4 weeks did not affect antipyrine or theophylline pharmacokinetics, suggesting that it does not interact with drugs metabolizing processes involving the P-450 microsomal system.
Other concomitant therapies
Although no specific policosanol research has been developed to evaluate its possible pharmacological interactions, in short and long-term clinical studies policosanol has been simultaneously employed each of the following drugs without evidence of clinically relevant adverse interactions.
- calcium antagonists,
- inhibitors of angiotensin-converting enzyme,
- beta-blockers,
- meprobamate,
- diuretics,
- nitroderivative vasodilators,
- non-steroidal anti-inflammatory drugs,
- anxiolytics,
- anti-depressant,
- neuroleptics, oral hypoglycemic agents,
- digoxin,
- warfarin,
- thyroid hormones,
- anti-ulcer drugs,
- between others
Policosanol Dosage
Although the tolerability profile remains excellent increasing the dose to 40 mg/day does not offer significant additional cholesterol-lowering benefits over the 20 mg/day dose.
SUMMARY OF POLICOSANOL RESEARCH
Policosanol is a mixture of fatty alcohols derived from the wax of sugar cane. These active substances work to lower cholesterol levels by several mechanisms. It inhibits cholesterol manufacturer but does so prior to HMG-CoA reductase. In addition policosanol also exerts exceptional effects on LDL-cholesterol metabolism.
Specifically, policosanol increases LDL receptor processing.It exerts this effect by increasing the binding of LDL to its receptor, improving the transport of LDL into the liver cell, and significantly enhancing the breakdown of LDL cholesterol.
In addition to lowering LDL, policosanol has also been shown to increase HDL, protect against free radical damage to LDL-cholesterol, and inhibit excessive platelet aggregation.
All together, policosanol exerts many pharmacological actions of benefit in the prevention and treatment of atherosclerosis or hardening of the arteries. The clinical documentation for policosanol is exceptional.
Well designed policosanol research studies trials have included short and long-term, randomized, double-blind studies comparing policosanol to a placebo as well as double-blind comparative trials versus statin drugs, fibrates, acipimox, and probucol.
Policosanol produces cholesterol-lowering effects within the first 6-8 weeks of use. At a daily dosage of 10-20 mg of policosanol at night, LDL cholesterol levels typically drop by 20 to 25% within the first six months of therapy.
At a dosage of 20 mg, LDL levels typically drop by 25-30%.
HDL cholesterol levels typically increase by 15 to 25% after only two months of use.
The combined LDL reduction and HDL increase can produce dramatic improvements in the LDL to HDL ratio.
Policosanol is completely safe.
Policosanol has not been shown to produce any drug interaction when used a wide range of concomitant therapies and has been used effectively in the following populations:
- diabetics,
- elderly subjects,
- patients with impaired liver function or severe liver damage without fear of side effects.
Policosanol is not only virtually side effect free it also has a number of health benefits, but the most powerful is its ability to increase your good cholesterol, which is more important than lowering your so called "bad" cholesterol.
Most statin drugs do NOT improve HDLs. HDL cholesterol levels typically increase by 15 to 25% after only two months of policosanol use.
The "good" HDL cholesterol is the "Superman" heart protector. HDL cholesterol acts like a miniature hydraulic vacuum cleaner, streaming through your arteries scooping up the heart- stopping LDL cholesterol, and carrying it back to your liver where it can be sent packing.
The benefits of policosanol are well documented by studies of nearly 30,000 persons. It comes with recommendations from recognized authorities such as Dr. Michael Murray. Unlike statin drugs such as Lipitor, Crestor and Zocor, side effects are rare and minimal.

I'm Gene Millen a Heart Health Coach and the co-owner of this website with my wife Bernie.
During the last 23 years I have worked with hundreds of people who are looking for ways to lower their cholesterol without the use of statin drugs.
I've invested untold hours studying the research to discover the best ways to keep my heart and (and yours) healthy.
It was nearly 13 years ago that I first discovered Policosanol. I've found Policosanol to be one of the best alternatives to Lipitor and other statin drugs as it lowers ldl cholesterol and increases the good hdl cholesterol...and without the side effects caused by statin drugs.
The following story is a powerful testimony of how Vital Life Nutritionals Policosanol Extra Strength Formula is working for Janelle.
"When the results of my cholesterol lab test came back in the spring of 2025, my triglycerides were sky high (1723) and my HDL cholesterol so low (8) that I was frightened enough to try any solution the doctor suggested.
"He prescribed a low-fat, low-carbohydrate diet, exercise and high doses of niacin. This brought the triglycerides down to a reasonable level; however, nothing seemed to budge the low HDL level to much above 18.
"When we moved out of state, my new doctor insisted that I try a combination of niacin and a statin drug to address what he thought were "unacceptable" numbers.
In 2025 I finally caved and agreed to the statin combination of niacin and a statin drug.
Within a year I was unable to walk up and down stairs and couldn't lift myself from a sitting position without holding on to something.
"I immediately stopped the statin/niacin (against the doctor's advice), and I began in earnest scouring the internet for alternative solutions to what for me had been harmful pharmaceutical drugs.
"How fortunate for me that I found the Millen's Vital Heart Health for Life site. I ordered policosanol and within six months my HDL began improving.
My last test, three months ago showed my HDL at 36, not ideal I realize, but for me the best it has ever been. Triglycerides and LDL were also within normal range, and my total cholesterol was 180.
"I am so very thankful that Gene and Bernie Millen continue to offer what I consider life-saving advice, and products such as policosanol, for those of us who are desperately searching for alternatives to the "traditional" drugs prescribed by most physicians today.
Janelle Carey, Savoy, Illinois
 Click here to try Vital Life Nutrionals Policosanol Extra Strength capsules (made with the same formula used in the clinical studies) Save up to 40%.
Click here to try Vital Life Nutrionals Policosanol Extra Strength capsules (made with the same formula used in the clinical studies) Save up to 40%.
Thanks for joining us on the journey to Vital Heart Health for Life!
Related Resources
Buy Policosanol Online
Policosanol Benefits
Policosanol Cholesterol
Recent Policosanol Research Trials
Policosanol Statin Drugs
Clinical Studies - References
Policosanol Research Reported by Dr. Teresa Hill in 20251.- Arruzazabala M. L., Carbajal D., Mas R., et al. (1994): Cholesterol-lowering effects of policosanol in rabbits. Biol. Res. 27:205-209.,
- Arruzazabala M. L., Valdes S., Mas R., et al. (1995): Effect of policosanol succesive dose increase in platelet aggregation healthy volunteers. Pharmacol. Res. 34:181-185.
- Valdes S., Arruzazabala M.L., Carbajal D., et al. (1996): Effect of policosanol on platelet aggregation in healthy volunteers. Intern. J.Clin. Pharmacol. Res. 16:67-72.
- Canetti M., Morera M., Illnait J., et al. (1995): One year study on the effect of policosanol (5 mg-twice-a-day) on lipid profile in patients with type II hypercholesterolemia.
- Adv. Ther.12:245-254.
- .
- Canetti M., Morera M., Illnait J., et al. (1995): A two year study on the efficacy and tolerability of policosanol in patients with type II hypercholesterolemia. Intern. J. Clin. Pharmacol. Res.
- 15:159-165
- Carbajal D, Arruzazabala M. L., Mas R., et al. (1994). Effects of policosanol on experimental thrombosis models. Prostaglandins Leuko. Essent. Fatty Acids 50:249-251.
- Noa M., Mas R. and Mesa A. del R. (1997): Effect of policosanol in circulating endothelial cell in experimental models in Sprague-Dawley rats and in rabbits. Br. J. Nutr. 49:999-1002.
- Batista J., Stusser I. L., Penichet M. and Uguet E.(1995): Doppler-ultrasound pilot studyof thecffects of long-term
- policosanol therapy on carotid-vertebral atherosclerosis. Curr. Ther.Res. 56:906-914.
- Fraga V., Menindez R., Anior A.M., et al. (1997): Effect of policosanol on in vivo and in vitro rat liver microsomal
- lipid pcroxidation. Arch. Medical Res. 28:355-360.
- Mas R., Menindez R., Fraga V., etal. (1997): Modification of rat lipoprotein peroxidation by oral administration of policosanol. Abstract from the
- 4th International Conference on Preventive Cardiology, June 29-July3.Can. J. Cardiol. 13:Suppl. B. 310B.
- Hernendez F., Illnait J., Mas R., et al. (1992): Effects of policosanol on serum lipids and lipoproteins in healthy volunteers. Curr. Ther. Res. 51:568-575. 21.Pons P., Mas R., Illnait J., et al. (1992): Efficacy and safety of policosanol in patients with primary hypercholesterolemia. Curr. Ther. Res. S2:507-513.
- Pons P., Rodriguez M., Robaina C., et al. (1994): Effects of succesive dose increases of policosanol on lipid profile of patients with type-II hypercholesterolemia and tolerability to treatment. J. Clin. Pharmacol. Res. 14:27-33.
- Aneiros E. Calderon B., Mas R., et al. (1993): Effects of successive dose increases of policosanol on the lipid profile and tolerability of treatment. Curr. Ther. Res. 54:304-312. 25. Aneiros E. Calderon B., Mas R., et al. (1995),
- Effect of policosanol in cholesterol-lowering levels in patients with type-II hypercholesterolemia. Curr. Ther. Res. 56:176-182. 26. Pons P.,Rodriguez M., Mas R., et al. (1994): One-year efficacy and safety of
- policosanol in patients with type-II hypercholesterolemia. Curr. Ther.Res. 55:1084-1092.
- Castano G., Mas R., Nodarse M., et al. (1995): One-year study of the efficacy and safety of policosanol (5 mg twice
- daily) in the treatment of type II hypercholesterolemia. Curr. Ther.Res., 56:296-304.
- Dalmer Laboratory. Policosanol vs lovastatin: Comparative study on efficacy, safety and tolerability in
- the treatment of type-II hypercholesterolemia. Data on file.
- Benitez M., Romero C., Mas R., et al. (1997): A comparative study of policosanol vs pravastatin in patients with type-II hypercholesterolemia. Curr. Ther. Res. 58:859-67.
- Ortensi G., Gladstein J., Vail H. and Tesone P.A. (1997): A comparativc study of policosanol vs. simvastatin in elderly patients with hypercholesterolemia. Curr. Ther. Res. 58:390-401.
- Illnait J., Castano G., Mas R. and Fernandez J.C. (1997): A comparative study on the efficacy and tolerability of policosanol and simvastatin for treating type II hypercholesterolemia. Abstract front the 4th International Confereiicc on Preventive Cardiology. June 29-July 3. Can. J. Cardiol. 13:Suppl. B, 342B. 36. Canetti M., Morera M., IllnaitJ., et al.
- Alcocer L, Campos A., Mas R. and Fernandez L. (1997): A comparative study of policosanol vs acipimox in patients with type II hypercholesterolemia. Data on file.
- Pons P., Illnait J., Mas R., et al. (1997): A comparativc study of policosanol versus probucol in patients with hypercholesterolemia. Curr. Ther. Res. 58:26-35.
- Torres O., Agramonte A. J., Illnait J., et al. (1995): Treatment of hypercholesterolemia in NIDDM with policosanol. Diabetes Care 18:393-397.
- Crespo N., Alvarez R., Mas R., et al. (1997): Effect of policosanol on patients with non-insulin-dependent diabetes mellitus and hypercholesterolemia: A pilot study. Curr. Ther. Res. 58:44-51. 43.Castano G., Tula L., Canetti M., et al.
- (1996): Effects of policosanol in hypertensive patients with type II hypercholesterolemia. Curr. Ther.
- Res. 57:691-699.
- Pons P., Jimenez A., Rodriguez M., et al. (1993):Effects of policosanol in elderly hypercholesterolemic patients. Curr.Ther. Res. 53:265-269. 45. Castano G., Canetti M., Morera M., et al.
- (1995): Efficacy and tolerability of policosanol in elderly patients with type-II hypercholesterolemia: A 12 months study. Curr. Ther. Res.56:819-828. 46. Zordoya R., Tula L., Castano G., Mas R., et al. (1996):
- Effects of policosanol on hypercholesterolemic patients with disturbances on serum biochemical indicators of hepatic function. Curr. Ther. Res. 57:568-577.
- Davalos J.M., Mederos H., Rodriguez J., et al. (1996): Effect of policosanol in hypercholesterolemia due to
- nephrotic syndrome. X Latinciamerican Congress of Nephrology and Hypertension, 14 September, Santiago de Chile, Chile.
- Arruzazabala M. L., Carbajal D., Mas R. and Valdes S. (1997): Comparative study of
- policosanol, aspirin and the combination therapy policosanol-aspirin on platelet aggregation in healthy volunteers. Pharmacol Res 36(4):293-7.
- Stusser R., Batista J., Padron R. et al. (1998): Long-term therapy with policosanol improves treadmill exercise-ECG testing performance of coronary heart disease patients. Int J Clin Pharmacol Ther 36(9):469-73.
- Fernandez L., Mas R., Illnait J., Fernandez J.C. (1998):Policosanol: Results of a postmarketing survellance study of 27,879 patients. Curr. Ther. Res. 59:7717-22.
- Aleman C.L., Mas R., Rodeiro L., et al. (1991): Toxicologia aguda
- del Ateromixol (PPG) en roedores. Rev. CENIC Cien. Biol. 22:102-105.
- Aleman C.L., Mas R., Rodeiro I., et al. (1992): Acute, subchronic and chronic toxicology of policosanol in rats. Toxicol. Letters. Suppl.2:248.
- Aleman C.L., Noa M., Cerejido E., Mis R., Rodeiro L Hcrnindez C. and Briffis F. (1995):
- Carcinogenicity of policosanol in mice: A 18 months study. Fd. and Client. Toxicol., 33:573-578.
- Aleman C.L., Mas R., Noa M., et al. (1994): Carcinogenicity of policosanol in Sprague Dawley rats: A 24
- months study. Teratog. Carcinog. and Mutag., 14:239-249. 58. Rodriguez M.D. and Garcia H. (1994): Teratogenic and reproductive studies of policosanol in the rat and rabbit. Teratog., Carcinog. and Mutag.,
- 14:107-113. <
- Rodriguez M.D., Garcia H. (1998): Evaluation of peri-and post-natal toxicity of Policosanol in rats. Teratog Carcinog
- Mutagen 18(1):1-7.
- Rodriguez M.D., Sanchez M., Garcia H. (1997): Multigeneration reproduction study of policosanol in rats. Toxicol.
- Lett. 90:97-106. 61. Carbajal D, Arruzazabala M. L., Mas R., et al.(1998): Interaction policosanol-warfarin on bleeding time and thrombosis in rats. Pharmacol Res 38(2):89-91.